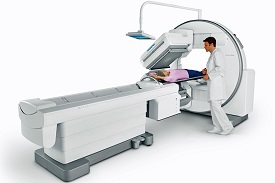
Specialized medical devices known as nuclear medicine accessories and equipment are used in the diagnosis and treatment of a wide range of medical illnesses, such as cancer, heart disease, and neurological disorders. The Indian Technical Standard MDW5 describes the manufacturing and quality requirements for these essential medical devices in India.
These standards give producers of nuclear medical equipment and accessories precise technical criteria, quality standards, safety precautions, and testing protocols to follow. Adhering to these guidelines can help manufacturers gain credibility with healthcare practitioners, as well as open doors for their products in the cutthroat medical equipment industry.
Note: Manufacturers of nuclear medicine accessories and equipment must first get an EPR Registration Certificate.
Specialized medical devices known as nuclear medicine accessories and equipment are used in the diagnosis and treatment of a wide range of medical illnesses, such as cancer, heart disease, and neurological disorders. The Indian Technical Standard MDW5 describes the manufacturing and quality requirements for these essential medical devices in India.
An essential program to handle the expanding problem of managing electronic trash, or "e-waste," is called EPRA, or Extended Producer's Responsibility Authorization. Securing EPR authorization from the State Pollution Control Boards (SPCBs) and receiving the EPR Registration Certificate from the Central Pollution Control Board (CPCB) are the two steps in the process of obtaining EPR authorization. With the help of EPRA, manufacturers and importers of electronic goods will take over management of e-waste from consumers and local governments. Electronics importers and manufacturers are obligated by EPRA to assume full responsibility for the whole lifecycle of their products, from manufacturing to disposal. Establishing and financing collection networks, recycling centers, and appropriate e-waste disposal techniques are all part of this.
For more detailed information, please click here.
Step 1: Fill out the application form
Step 2: Attach all the required documents
For more detailed information, please click here
Brand Liaison offers valuable assistance in obtaining an EPR Registration Certificate for Nuclear Medicine Equipment and accessories, Our services include:
For a more in-depth understanding of the documentation and procedures involved in obtaining EPR Registration, feel free to connect with our team of experts at +91-9250056788 or +91-8130856678.
Back